Metal coordination complexes have a wide variety of technological and industrial application ranging from catalysis to anticancer drugs. The influence of transition metals in biological systems had already long been recognized. Metal ions are essential to human health. Excessive quantities of metal ions are linked to a number of harmful side effects, including cancer. These factors make coordination metal complexes as medications one of the most alluring and fascinating fields in medical chemistry.
1. INTRODUCTION
1.1 Transition Elements
Transition elements (also known as transition metals) are elements that have partially filled d orbitals. IUPAC defines transition elements as an element having a d subshell that is partially filled with electrons, or an element that has the ability to form stable cations with an incompletely filled d orbital. In general, any element which corresponds to the d-block of the modern periodic table (which consists of groups 3-12) is considered to be a transition element. Even the f-block elements comprising the lanthanides and the actinides can be considered as transition metals. However, since the f-block elements have incompletely filled f-orbitals, they are often referred to as inner transition elements or inner transition metals.
Metal ions and their function in biological systems have long been understood. While some metals are deemed hazardous, others are desperately needed. Iron (Fe2+/3+) is actually the most significant metal in transition metals. [1-6] Life wouldn’t be possible without iron. A necessary trace element found in nature as Cu2+, copper is involved in metalloproteins such as corbate oxidase, superoxide dismutase, and cytochrome oxidase. We also know that Mn is involved in photosynthesis, that Co is involved in vitamin B12, and that Ni is involved in urease. One possible function of chromium (III) is in the metabolism of glucose. However, the harmful effects of Cr (VI) are thought to be carcinogenic. Metal ions are essential to human health. Diseases like as anemia through iron deficiency, development retardation from inadequate zinc intake, and baby cardiac disease from copper deficiency can be caused by a lack of certain metal ions. Both medications and diagnostic tools require metal ions. [7–12] Among the special qualities that metals possess are redox activity, coordination sites, and reactivity with organic groups. Excessive quantities of metal ions are linked to a number of harmful side effects, including cancer. These factors make coordination metal complexes as medications one of the most alluring and fascinating fields in medical chemistry. [13–15] Many biological processes in nature heavily rely on metal ions like copper and zinc, which are essential to the regular operation of living things. [1–15] Metal coordination complexes have a wide variety of technological and industrial application ranging from catalysis to anticancer drugs. In these compounds the metal atom itself may have a number of roles, based on its coordination geometry, oxidation state and magnetic electronic and photochemical behaviors. The coordination chemistry of any transition metal seems to be a complicated function that involves numerous variables. Schiff bases are an important class of ligands in coordination chemistry and their complexing ability containing different donor atoms are widely reported [16-20] Medicinal inorganic chemistry as a discipline is considered to have boosted with the discovery of the anticancer properties of cisplatin. Thus the application of inorganic chemistry to medicine is a rapidly developing field, and novel therapeutic and diagnostic metal complexes are now having an impact on medicinal practice.[21] significant structural differences between ruthenium and platinum-based antitumor drugs; yet ruthenium based drugs could be suitable alternatives to cis-platin and carbo With the new emerging fields of science viz.; genetic engineering molecular biology, cell biology, nano science, biotechnology, magnetic resonance imaging bioinformatics etc. The intellectual ability of the chemist plays a pivotal role in the development of new bioactive molecules. The primary task of the chemist is to prepare specific new molecules that can lead more efficiently to useful drug discovery. The preparation of a new ligand was perhaps the most important step in the development of metal complexes which exhibit unique properties and novel reactivity. Since the electron donor and electron acceptor properties of the ligand, structural function groups and the position of the ligand in the coordination sphere together with the reactivity of coordination compounds may be the factor for different studies [27] interest stems from the ease with which they can be synthesized, due to their versatility a range of complexing ability. Owing to large considered to have boosted with the discovery of the anticancer properties of cisplatin. Thus, the chemistry to medicine is a rapidly developing field,
and novel therapeutic and diagnostic metal complexes are now having an impact on medicinal practice. [22-24].There are significant structural differences between based antitumor drugs; yet ruthenium based drugs could be suitable platin and carbo-platin.[25-26]. With the new emerging fields of science viz.; genetic engineering molecular biology, cell biology, nano science, biotechnology, magnetic formatics etc. The intellectual ability of the chemist plays a pivotal role in the development of new bioactive molecules. The primary task of the chemist is to prepare specific new molecules that can lead more efficiently to useful drug discovery. The paration of a new ligand was perhaps the most important step in the development of metal complexes which exhibit unique properties and novel reactivity. Since the electron donor and electron acceptor properties of the ligand, the position of the ligand in the coordination sphere together with the reactivity of coordination compounds may be [27-29]. Their interest stems from the ease with which they can be synthesized, due to their versatility and wide variety of coordination geometries, coordination number and modes of interaction with their ligands, metal complexes give access to different field of pathways in cancer treatment than do organic compounds. The metal complexes of transition element with heterocyclic ligands, especially those containing nitrogen and Sulphur have diverse applications in various fields including biology and anti- herbicidal activities of thioamide ligands and its metal complexes are well known and get more attraction recently.
Sulphur and nitrogen donor ligands are also used as powerful pesticides. The documented biological activities of heterocyclic ligands as well as their metal complexes have attracted much attention over the years. Numbers of heterocyclic compounds are well known and such activities have often been related to their chelating abilities towards one more essential trace metal ions. Being fascinated by significant biological implications of transition metal complexes of various Schiff bases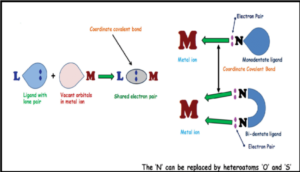
1. INTRODUCTION
1.1 Transition Elements
Transition elements (also known as transition metals) are elements that have partially filled d orbitals. IUPAC defines transition elements as an element having a d subshell that is partially filled with electrons, or an element that has the ability to form stable cations with an incompletely filled d orbital. In general, any element which corresponds to the d-block of the modern periodic table (which consists of groups 3-12) is considered to be a transition element. Even the f-block elements comprising the lanthanides and the actinides can be considered as transition metals. However, since the f-block elements have incompletely filled f-orbitals, they are often referred to as inner transition elements or inner transition metals.
Metal ions and their function in biological systems have long been understood. While some metals are deemed hazardous, others are desperately needed. Iron (Fe2+/3+) is actually the most significant metal in transition metals. [1-6] Life wouldn’t be possible without iron. A necessary trace element found in nature as Cu2+, copper is involved in metalloproteins such as corbate oxidase, superoxide dismutase, and cytochrome oxidase. We also know that Mn is involved in photosynthesis, that Co is involved in vitamin B12, and that Ni is involved in urease. One possible function of chromium (III) is in the metabolism of glucose. However, the harmful effects of Cr (VI) are thought to be carcinogenic. Metal ions are essential to human health. Diseases like as anemia through iron deficiency, development retardation from inadequate zinc intake, and baby cardiac disease from copper deficiency can be caused by a lack of certain metal ions. Both medications and diagnostic tools require metal ions. [7–12] Among the special qualities that metals possess are redox activity, coordination sites, and reactivity with organic groups. Excessive quantities of metal ions are linked to a number of harmful side effects, including cancer. These factors make coordination metal complexes as medications one of the most alluring and fascinating fields in medical chemistry. [13–15] Many biological processes in nature heavily rely on metal ions like copper and zinc, which are essential to the regular operation of living things. [1–15] Metal coordination complexes have a wide variety of technological and industrial application ranging from catalysis to anticancer drugs. In these compounds the metal atom itself may have a number of roles, based on its coordination geometry, oxidation state and magnetic electronic and photochemical behaviors. The coordination chemistry of any transition metal seems to be a complicated function that involves numerous variables. Schiff bases are an important class of ligands in coordination chemistry and their complexing ability containing different donor atoms are widely reported [16-20] Medicinal inorganic chemistry as a discipline is considered to have boosted with the discovery of the anticancer properties of cisplatin. Thus the application of inorganic chemistry to medicine is a rapidly developing field, and novel therapeutic and diagnostic metal complexes are now having an impact on medicinal practice.[21] significant structural differences between ruthenium and platinum-based antitumor drugs; yet ruthenium based drugs could be suitable alternatives to cis-platin and carbo With the new emerging fields of science viz.; genetic engineering molecular biology, cell biology, nano science, biotechnology, magnetic resonance imaging bioinformatics etc. The intellectual ability of the chemist plays a pivotal role in the development of new bioactive molecules. The primary task of the chemist is to prepare specific new molecules that can lead more efficiently to useful drug discovery. The preparation of a new ligand was perhaps the most important step in the development of metal complexes which exhibit unique properties and novel reactivity. Since the electron donor and electron acceptor properties of the ligand, structural function groups and the position of the ligand in the coordination sphere together with the reactivity of coordination compounds may be the factor for different studies [27] interest stems from the ease with which they can be synthesized, due to their versatility a range of complexing ability. Owing to large considered to have boosted with the discovery of the anticancer properties of cisplatin. Thus, the chemistry to medicine is a rapidly developing field,
and novel therapeutic and diagnostic metal complexes are now having an impact on medicinal practice. [22-24].There are significant structural differences between based antitumor drugs; yet ruthenium based drugs could be suitable platin and carbo-platin.[25-26]. With the new emerging fields of science viz.; genetic engineering molecular biology, cell biology, nano science, biotechnology, magnetic formatics etc. The intellectual ability of the chemist plays a pivotal role in the development of new bioactive molecules. The primary task of the chemist is to prepare specific new molecules that can lead more efficiently to useful drug discovery. The paration of a new ligand was perhaps the most important step in the development of metal complexes which exhibit unique properties and novel reactivity. Since the electron donor and electron acceptor properties of the ligand, the position of the ligand in the coordination sphere together with the reactivity of coordination compounds may be [27-29]. Their interest stems from the ease with which they can be synthesized, due to their versatility and wide variety of coordination geometries, coordination number and modes of interaction with their ligands, metal complexes give access to different field of pathways in cancer treatment than do organic compounds. The metal complexes of transition element with heterocyclic ligands, especially those containing nitrogen and Sulphur have diverse applications in various fields including biology and anti- herbicidal activities of thioamide ligands and its metal complexes are well known and get more attraction recently.
Sulphur and nitrogen donor ligands are also used as powerful pesticides. The documented biological activities of heterocyclic ligands as well as their metal complexes have attracted much attention over the years. Numbers of heterocyclic compounds are well known and such activities have often been related to their chelating abilities towards one more essential trace metal ions. Being fascinated by significant biological implications of transition metal complexes of various Schiff bases

2. CLASSES OF METAL BASED PHARMACEUTICALS
There are various classes of metal-based pharmaceuticals that can be divided into seven groups depending on which part of the structure is responsible for the biological activity of the compound
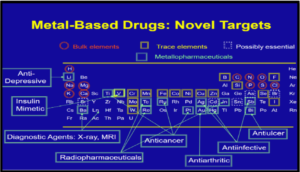
There are various classes of metal-based pharmaceuticals that can be divided into seven groups depending on which part of the structure is responsible for the biological activity of the compound

a. Entire inert complex is active
Here the complexes are synthesized from smaller and simpler components. An example is the group of ruthenium complexes that are highly potent inhibitors of protein kinases [30].
b. Entire reactive complex is active
The copper complexes of NSAIDs that can mimic the actions of some enzymes, for instance superoxide dismutase, and have an analgesic effect. Very active complexes are the macrocyclic [31] and porphyrin [32] Mn(II) complexes. A five-coordinate intermediate is probably involved in the mechanism of action of the macrocylic complex.
c. A fragment of the complex is active
These are of special importance because the non-leaving ligands can mediate the interaction with the target and give selective activity of Platinum (II) complexes such as cisplatin are representatives of this group because they lose the anionic ligands (i.e., chloride) and form coordinate bonds with DNA. (To be discussed in detail below.) However, their toxic side effects have been a problem and require development of new less toxic prodrugs in form of platinum (IV) complexes [33]. Yet, another use of platinum (IV)complexes is their activation on exposure to light [34].
3. THE METAL ION OR ONE OF ITS BIOTRANSFORMATION
PRODUCTS IS ACTIVE
The complexes that deliver an active metal are for instance insulin potentiating vanadium complexes. Orthovanadate mimics phosphate and inhibits the protein tyrosine phosphatases causing an increased cellular uptake of insulin [35]. However, the problem is with the bioavailability from the gastric system. One of the maltolato complexes of vanadium (IV), bis(ethylmaltolato)oxovanadium (IV), appeared to be effective and entered phase I of the human trials with no adverse effects [36]. One of the least toxic transition metals it is unfortunately taken up and stored in bones, resulting in side effects.
Here the complexes are synthesized from smaller and simpler components. An example is the group of ruthenium complexes that are highly potent inhibitors of protein kinases [30].
b. Entire reactive complex is active
The copper complexes of NSAIDs that can mimic the actions of some enzymes, for instance superoxide dismutase, and have an analgesic effect. Very active complexes are the macrocyclic [31] and porphyrin [32] Mn(II) complexes. A five-coordinate intermediate is probably involved in the mechanism of action of the macrocylic complex.
c. A fragment of the complex is active
These are of special importance because the non-leaving ligands can mediate the interaction with the target and give selective activity of Platinum (II) complexes such as cisplatin are representatives of this group because they lose the anionic ligands (i.e., chloride) and form coordinate bonds with DNA. (To be discussed in detail below.) However, their toxic side effects have been a problem and require development of new less toxic prodrugs in form of platinum (IV) complexes [33]. Yet, another use of platinum (IV)complexes is their activation on exposure to light [34].
3. THE METAL ION OR ONE OF ITS BIOTRANSFORMATION
PRODUCTS IS ACTIVE
The complexes that deliver an active metal are for instance insulin potentiating vanadium complexes. Orthovanadate mimics phosphate and inhibits the protein tyrosine phosphatases causing an increased cellular uptake of insulin [35]. However, the problem is with the bioavailability from the gastric system. One of the maltolato complexes of vanadium (IV), bis(ethylmaltolato)oxovanadium (IV), appeared to be effective and entered phase I of the human trials with no adverse effects [36]. One of the least toxic transition metals it is unfortunately taken up and stored in bones, resulting in side effects.
4. ANTIINFECTIVE METAL ION COMPLEXES
Aside from drugs that fight cancer, anti-infective therapy presents another therapeutic challenge in medicine. There are numerous compounds that are effective against viruses, fungi, or bacteria, but due to rising drug resistance, even the best antibiotics may not be able to treat illnesses. As a result, a lot of work is put into creating compounds with different chemical structures that are more effective than the current medications available on the market to treat bacterial infections.
Inorganic mercury salts were among the first antibacterial substances to be used. Nevertheless, the compounds’ only action was bacteriostatic. The substances inhibited the growth of bacterial enzymes by binding to their sulfhydryl groups. Additionally, silver is utilized in forms other than silver nitrate and possesses strong antibacterial properties. [37] Antibacterial therapy aims among others at interfering in the biosynthesis of bacterial cell wall or the synthesis of the bacterial DNA or proteins. Apart from zinc (II) pyrithione, which is used in the anti-dandruff shampoos, so far there are no copper (II), cobalt (II) or platinum (II) complexes used
therapeutically. Nevertheless, there is much research going into finding a good agent that could be of better activity than known antibiotics. Most of all, much attention was paid to metal complexes of sulfonamides [38-42] and benzimidazoles [43-46].
5. BIOLOGICAL PROPERTIES OF COPPER
Since copper is a necessary element for life, it has received a lot of attention. It is connected to several copper-dependent vital enzymes for biological processes. Mammals’ most significant copper-dependent enzymes [47, 48]. Elevated copper levels in plasma can be important for the etiology of some illness [49]. For example, copper ions are closely involved in neurodegenerative disorders [50-52], especially in Parkinson’s disease [53-54]. Moreover, there has been interest in the medical uses of copper, in particular as a complexing ion of known biologically active ligands and drugs. Throughout the years of scientific research copper (II) complexes have been found to possess various activities such as antiulcer [55], anti-amoebic [56], antidiabetic [57] anticonvulsant [58], anti-inflammatory [59-61], antimicrobial [62] and antitumor [63]. In particular, anti-inflammatory, anti-microbial and anti-cancer activity of copper complexes has been studied. The
three sub-chapters below briefly review copper complexes with such pharmaceutical properties. Another interesting compound is the copper (II) complex of 3,5diisopropylsalicylic acid, [Cu(II)(3,5-Dips)2]2 that shows not only anti-inflammatory but also antiulcer, anticarcinogenic, anticonvulsant, antidiabetic and analgesic properties [64-67].
Aside from drugs that fight cancer, anti-infective therapy presents another therapeutic challenge in medicine. There are numerous compounds that are effective against viruses, fungi, or bacteria, but due to rising drug resistance, even the best antibiotics may not be able to treat illnesses. As a result, a lot of work is put into creating compounds with different chemical structures that are more effective than the current medications available on the market to treat bacterial infections.
Inorganic mercury salts were among the first antibacterial substances to be used. Nevertheless, the compounds’ only action was bacteriostatic. The substances inhibited the growth of bacterial enzymes by binding to their sulfhydryl groups. Additionally, silver is utilized in forms other than silver nitrate and possesses strong antibacterial properties. [37] Antibacterial therapy aims among others at interfering in the biosynthesis of bacterial cell wall or the synthesis of the bacterial DNA or proteins. Apart from zinc (II) pyrithione, which is used in the anti-dandruff shampoos, so far there are no copper (II), cobalt (II) or platinum (II) complexes used
therapeutically. Nevertheless, there is much research going into finding a good agent that could be of better activity than known antibiotics. Most of all, much attention was paid to metal complexes of sulfonamides [38-42] and benzimidazoles [43-46].
5. BIOLOGICAL PROPERTIES OF COPPER
Since copper is a necessary element for life, it has received a lot of attention. It is connected to several copper-dependent vital enzymes for biological processes. Mammals’ most significant copper-dependent enzymes [47, 48]. Elevated copper levels in plasma can be important for the etiology of some illness [49]. For example, copper ions are closely involved in neurodegenerative disorders [50-52], especially in Parkinson’s disease [53-54]. Moreover, there has been interest in the medical uses of copper, in particular as a complexing ion of known biologically active ligands and drugs. Throughout the years of scientific research copper (II) complexes have been found to possess various activities such as antiulcer [55], anti-amoebic [56], antidiabetic [57] anticonvulsant [58], anti-inflammatory [59-61], antimicrobial [62] and antitumor [63]. In particular, anti-inflammatory, anti-microbial and anti-cancer activity of copper complexes has been studied. The
three sub-chapters below briefly review copper complexes with such pharmaceutical properties. Another interesting compound is the copper (II) complex of 3,5diisopropylsalicylic acid, [Cu(II)(3,5-Dips)2]2 that shows not only anti-inflammatory but also antiulcer, anticarcinogenic, anticonvulsant, antidiabetic and analgesic properties [64-67].

6. BIOLOGICAL PROPERTIES OF COBALT
The word ‘cobalt’ is derived from the German ‘kobalt’, from kobold, meaning ‘goblin’, a word used by miners for the ore of cobalt [68]. Cobalt like copper is an essential trace element for higher organisms. It is required in the active center of coenzymes, the so-called cobalamins (especially Vitamin B12 which regulates indirectly the synthesis of DNA). Moreover, there are at least eight cobalt-dependent proteins. Cobalamins alone are pharmaceutical agents and are treated in
pathologies arising from a lack of vitamin B12 [69]. The cobalt complexes are of more limited medical usage compared to copper complexes. Since the first reported studies on the biological activity of cobalt complexes in 1952 [70], there has been interest in cobalt(III) complexes of bidentate mustards, which appear to act as hypoxia-selective agents [71]. Some complexes have already been found active not only against leukemia and lymphoma cell lines [72] but also against
bacteria strains [73]. Furthermore, cobalt complexes possess in vivo insulin-like properties [74], antifungal [75] and antioxidant activity [76].
7. BIOLOGICAL PROPERTIES OF ZINC
Zinc is an essential trace element. It is found throughout the human body in a variety of tissues, such as skin, bone, liver, muscle or brain. In fact, this element is the most abundant transition metal in the brain after iron [77], and concentrations of zinc may reach 0.1-0.5 mm in the gray matter of the brain. It is also important for their biological activity as a constituent of proteins and enzymes that belong to cellular signaling pathways. Not only is it essential for the folding of DNA-binding
domains of transcription factors (zinc-finger and hormone receptor families) [78] but zinc also has a variety of effects on the nervous system. It plays a crucial role in regulating the aspects of cellular metabolism, including protein, hormone, transcription and replication functions [79]. However, overabundant levels of zinc can lead to apoptosis and neuronal death [80]. It is to regulate the zinc balance in the body to maintain the homeostasis [81]. Both zinc overload and deficiency lead to
pathologic processes in the central nervous system [82]. For example, zinc deficiency has been noticed in patients with head and neck cancer, It has also been associated with increased tumor size and the overall stage of the cancer [83].
The word ‘cobalt’ is derived from the German ‘kobalt’, from kobold, meaning ‘goblin’, a word used by miners for the ore of cobalt [68]. Cobalt like copper is an essential trace element for higher organisms. It is required in the active center of coenzymes, the so-called cobalamins (especially Vitamin B12 which regulates indirectly the synthesis of DNA). Moreover, there are at least eight cobalt-dependent proteins. Cobalamins alone are pharmaceutical agents and are treated in
pathologies arising from a lack of vitamin B12 [69]. The cobalt complexes are of more limited medical usage compared to copper complexes. Since the first reported studies on the biological activity of cobalt complexes in 1952 [70], there has been interest in cobalt(III) complexes of bidentate mustards, which appear to act as hypoxia-selective agents [71]. Some complexes have already been found active not only against leukemia and lymphoma cell lines [72] but also against
bacteria strains [73]. Furthermore, cobalt complexes possess in vivo insulin-like properties [74], antifungal [75] and antioxidant activity [76].
7. BIOLOGICAL PROPERTIES OF ZINC
Zinc is an essential trace element. It is found throughout the human body in a variety of tissues, such as skin, bone, liver, muscle or brain. In fact, this element is the most abundant transition metal in the brain after iron [77], and concentrations of zinc may reach 0.1-0.5 mm in the gray matter of the brain. It is also important for their biological activity as a constituent of proteins and enzymes that belong to cellular signaling pathways. Not only is it essential for the folding of DNA-binding
domains of transcription factors (zinc-finger and hormone receptor families) [78] but zinc also has a variety of effects on the nervous system. It plays a crucial role in regulating the aspects of cellular metabolism, including protein, hormone, transcription and replication functions [79]. However, overabundant levels of zinc can lead to apoptosis and neuronal death [80]. It is to regulate the zinc balance in the body to maintain the homeostasis [81]. Both zinc overload and deficiency lead to
pathologic processes in the central nervous system [82]. For example, zinc deficiency has been noticed in patients with head and neck cancer, It has also been associated with increased tumor size and the overall stage of the cancer [83].

A replenishment of zinc induced apoptosis in esophageal epithelial cells thus reducing the growth of the cancer [84]. Yet another way of action for zinc may be its effect on angiogenesis, which plays a critical role in carcinogenesis and tumors progression [85]. Elevated extracellular levels of zinc lead to the breakdown of the zinc transporting system of the plasma membrane. The resulting enhanced intracellular zinc concentration activates apoptosis [86]. However, an increased
apoptosis in vivo may be a consequence of a decrease in zinc intracellular concentrations. Therefore, cellular zinc is also described as an inhibitor of apoptosis because its depletion causes death in cell lines [87]. A good antimicrobial and anti-inflammatory agent, zinc has also been successfully tested for healing zinc-deficient, chronic and surgical wounds by local administration [88]. In the literature, there are many zinc complexes reported but only these associated with drugs
in the treatment of Alzheimer’s disease [89] or showing antimicrobial [90], anticonvulsant [91], anti-inflammatory [92], antidiabetic [93] or antitumor [94-96] activities are structurally described.
CONCLUSION
Transition metals are generally contained in organometallic complexes in our bodies, which reduces their tendency to encourage the production of hazardous reactive oxygen species and allows their characteristics to be regulated and directed where necessary. The oxygen transport proteins hemoglobin and hemocyanin, as well as a large number of enzymes and electron transport proteins, depend on transition metals. Many transcription factors use zinc finger motifs as their DNA-binding domains, and many enzymes involved in DNA replication and repair contain FeS clusters. Deficits of these metals caused by diet or genetics are linked to a number of pathologic conditions, such as sulfite oxidase deficiency (Mo), Menkes disease (Cu), and pernicious anemia (Fe). The majority of heavy metals, including some of the nutritionally important transition metals, are extremely toxic and almost all have the potential to cause cancer when consumed in high concentrations [97]
apoptosis in vivo may be a consequence of a decrease in zinc intracellular concentrations. Therefore, cellular zinc is also described as an inhibitor of apoptosis because its depletion causes death in cell lines [87]. A good antimicrobial and anti-inflammatory agent, zinc has also been successfully tested for healing zinc-deficient, chronic and surgical wounds by local administration [88]. In the literature, there are many zinc complexes reported but only these associated with drugs
in the treatment of Alzheimer’s disease [89] or showing antimicrobial [90], anticonvulsant [91], anti-inflammatory [92], antidiabetic [93] or antitumor [94-96] activities are structurally described.
CONCLUSION
Transition metals are generally contained in organometallic complexes in our bodies, which reduces their tendency to encourage the production of hazardous reactive oxygen species and allows their characteristics to be regulated and directed where necessary. The oxygen transport proteins hemoglobin and hemocyanin, as well as a large number of enzymes and electron transport proteins, depend on transition metals. Many transcription factors use zinc finger motifs as their DNA-binding domains, and many enzymes involved in DNA replication and repair contain FeS clusters. Deficits of these metals caused by diet or genetics are linked to a number of pathologic conditions, such as sulfite oxidase deficiency (Mo), Menkes disease (Cu), and pernicious anemia (Fe). The majority of heavy metals, including some of the nutritionally important transition metals, are extremely toxic and almost all have the potential to cause cancer when consumed in high concentrations [97]
REFERENCES
1. Collman JP, Gagne RR, Reed CA, Halbert TR, Lang G, Robinson WT, et al. Picket fence porphyrins. Synthetic models for oxygen binding hemoproteins. J Am Chem Soc. 1975;97:1427–39.
2. Schroeder HA, Balassa JJ, Tipton IH. Abnormal trace metals in man — nickel. J Chronic Dis. 1962;15(1):51–65.
3. Robert A, Eberle D, Kaplocoitz N. Role of glutathione in gastric mucosal cytoprotection. Am JPhysiol. 1984;247(3 Pt 1):296–304.
4. Hay RW. Inorganica Chimica Acta. Inorg Chim Acta. 1980;45:L83–4.5. Spike CG, Parry RW. Thermodynamics of Chelation. II. Bond Energy Effects in Chelate Ring Formation. J Am Chem Soc. 1953;75(15):3770–2.
6. Kasim ANM, Prabhu GV. Synthesis, Characterisation and Antimicrobial Screening of Aminobenzylated Mannich Bases of Acetamide and Urea. Asian J Chem. 2000;12(2):385–8.
7. Seigel H, Martin RB. Coordinating properties of the amide bond. Stability and structure of metal ion complexes of peptides and related ligands. Chem Rev. 1982;82(4):385–426.
8. Dyakar G, Lingaiah P. Synthesis and spectral studies of Ruthenium (III) complexes with amide group ligands. Asian J Chem. 1997;9(2):179–82.
9. Dyakar G, Lingaiah P. Studies on rhodium (III) complexes with ligands containing amide group. Indian J Chem. 1996;35A:614–6.
10. Senti F, Harker D. The Crystal Structure of Rhombohedral Acetamide. J Am Chem Soc. 1940;62(8):2008–2019.
11. Gerrard W, Lappert MF, Pyszora H, Wallis JW. Spectra and structure of amide complexes. J Chem Soc. 1960;p. 2144–51.
12. Kutzclnigg W, Mecke R. Spektroskopische Untersuchungen an organischen Ionen—V Die Struktur der Salze des Acetamids. Spectrochim Acta. 1962;18(4):549–60.
13. Penland RB, Mizushima S, Curran C, Quagliano JV. Infrared Absorption Spectra of Inorganic Coördination Complexes. X. Studies of Some Metal-Urea Complexes. J Am Chem Soc. 1957;79(7):1575–8.
14. Lippard SJ, Berg JM. Principles of Bioinorganic Chemistry. Mill Valley, CA: University Science Books; 1994.
15. Cowan JA. Inorganic Biochemistry/An introduction. Hoboken, NJ: Wiley-VCH Inc; 1997.
16. Wilkinson G, Gillard RD, Mc Cleverty JD. (Ed.) “Comprehensive coordination chemistry”, Pergamon, Oxford; 1987.
17. Mc Cleverty JA, Meyer TJ, “Comprehensive coordination chemistry II, from biology to nanotechnology” Elsevier, Amsterdam; 2003.
18. Cotton FA, Wilkinson G, Murillo CA, Bochmann M, “Advanced inorganic chemistry” 6th Edn., John Wiley and Sons, Inc; 1999.
19. Samy CR, Radhey S. Indian J. Chem. 1996;35A:1.
20. Shen X, Yang QLC, Xie Synth. React. Inorg. Met. Org. Chem. 1996;26:1135.
21. Kostova I. Recent patents on anti-cancer drug discovery. 2006;1:1.
22. Kostova I. Recent patents on anti-cancer drug discovery. 2006;1:1.
23. Allardyce CS, Dyson PJ. Platinum Metals Rev. 2001;45:62.
24. Abu-Surrah AS, Kettunen M. curr. Med. Chem. 2006;13:1337. Khan et al.; IRJPAC, 22(5): 12-23, 2021.
25. Clarke MJ. Coord. Chem Rev. 2003;23:209.
26. Zhang CX, Lippard SJ. Current Opinion in Chem. Bio. 2003;7:481.
27. Lions F, Martin FKV. J. Am. Chem. Soc. 1960;82:2733.
28. Greenwood NN, Earnshaw A, “Chemistry of Elements”, Pergamon; 1985.
29. Gerloch M, EC. Constable, “Transition metal chemistry”, VCH, Weinheim; 1994.
30. Clark MJ, Zhu F, Frasca DR. Chem. Rev. 1999;9:2511.
31. Guo Z, Sadler PJ. Angew Chem., Int Ed. 1999;11:1512.
32. Allardyce CS, Dorcier A, Scolaro C, Dyson PJ. Appl. Organomet. Chem. 2005;19:1.
33. Guo Z, Sadler PJ, Sykes AG. Adv. Inorg. Chem. 1999;49:183.
34. Di C, Milacic V, Frezza M, Ping Dou Q. Curr. Pharm. Des. 2009;7:777.
35. Bruijnincx PCA, Sadler PJ. Curr. Opin. Chem. Biol. 2008;2:197.
36. Dyson PJ, Sava G. Dalton Trans. 2006;16:1929.
37. Keppler BK, Berger MR, Klenner T, Heim ME. Adv. Drug. Res. 1990;19:243
38. Chohan ZH, Munawar A, Supuran CT. Metal Based Drugs. 2001;8:137.
39. Srivastava A, Singh NK, Singh SM. Biometals. 2003;16:311.
40. Hambley TW. Developing new metalbased therapeutics: Challenges and opportunities. Dalton Trans. 2007;43:4929- 4937.
41. Keppler BK, Berger MR, Klenner Th, Heim ME. Metal complexes as antitumor agents. Adv. Drug. Res. 1990;19:243.
42. Bregman H, Carroll PJ, Meggers E. Rapid access to unexplored chemical space by ligand scanning around a ruthenium center: Discovery of potent and selective protein kinase inhibitors. J. Am. Chem. Soc. 2006;128:877-884.
43. Riley DP, Lennon PJ, Neumann WL, Weiss RH. Toward the rational design of superoxide dismutase mimics: Mechanistic studies for the elucidation of substituent effects on the catalytic activity of macrocyclic manganese (II) complexes. J. Am. Chem. Soc. 1997;119:6522-5528.
44. Pacher P, Liaudet L, Bai PP, Mabley JG, Kaminski PM, Virag A, Deb L, Szabo E, Ungvari Wolin MS, Groves JT, Szabo C. Potent metalloporphyri peroxynitrite decomposition catalyst protects against the development of doxorubicininduced cardiac dysfunction. Circulation. 2003;107:896-904.
45. Hall MD, Dolman RC, Hambley TW. In metal complexes in tumor diagnosis and as anticancer agents, metal ions in biological systems, ed. A. Sigel and H. Sigel, Marcel Dekker, Inc., New York and Basel. 2004;24:297-322.
46. Bednarski PJ, Grunert R, Zielzki M, Wellner A, Mackay FS, Sadler PJ. Light activated destruction of cancer cell nuclei by platinum diazide complexes. Chem. Biol. 2006;13:61-67.
47. Fantus IG, Deragon G, Lai R, Tang S. Modulation of insulin action by vanadate: Evidence of a role for phosphotyrosine phosphatase activity to alter cellular signaling. Mol.Cell. Biochem. 1995;153:103-112.
48. Thompson TH, Orvig C. Vanadium in diabetes: 100 years from Phase 0 to Phase I. J. Inorg. Biochem. 2006;100:1925-1935.
49. Sessler JL, Seidel D. Synthetic expanded porphyrin chemistry. Angew. Chem. Int. Edit. 2003;42:5134-5175.
50. Hutchings SR , Song DZ, Fricker SP, Pang CCY. The ruthenium-based nitric oxide scavenger, AMD6221, augments cardiovascular responsiveness to noradrenaline in rats with streptozotocininduced diabetes. Eur. J. Pharmacol. 2005;528:132-136.
51. Musameh MD, Fuller BJ, Mann BE, Green CJ, Motterlini R. Positive inotropic effects of carbon monoxide-releasing molecules (CO-RMs) in the isolated perfused rat heart. Br. J.Pharmacol. 2006;149:1104- 1112.
52. Failes TW, Cullinane C, Diakos CI, Yamamoto N, Lyons JG, Hambley TW. Studies of a cobalt(III) complex of the MMP inhibitor marimastat: A potential hypoxiaactivated prodrug.Chem. Eur. J. 2007;13:2974-2982.
53. Weder JE, Dillon CT, Hambley TW, Kennedy BJ, Lay PA, Biffin JR, Regtop HL, Davies NM. Copper complexes of nonsteroidal anti-inflammatory drugs: an opportunity yet to be realized. Coord. Chem. Rev. 2002;232:95-126.
54. Kostowski W, Herman ZS. Farmakologia, Podstawyfarma, koterapiiWydawnic, lekarskie PZWL, Warszawa, Poland. 2003;62:270-272.
55. Blasco F, Perello L, Latorre J, Borras J, Garcia-GrandaCobalt S (II), Nikiel(II), Copper (II) complexes of sulfanilamide derivatives: Synthesis, spectroscopic studies, and antibacterial activity. Crystal structure of [Co(sulfacetamide) 2 (NCS)2]. J. Inorg. Biochem. 1996;61:143- 154.
56. Kremer E, Facchin G, Estevez E, Albores P, Baran EJ, Ellena J, Torre MH. Copper complexes with heterocyclic sulfonamides: Synthesis, spectroscopic characterization, microbiological and SOD-like activities: Crystal structure of [Cu(sulfisoxazole)2(H2O)4]*2H2O. J. Inorg. Biochem. 2006;100:1167-1175.
57. Olar R, Badea M, Carp O, Marinescu D, Lazar V, Balotescu C, Dumbrava A. Synthesis, characterisation and thermal behaviour of some thiosulfato- and sulfato copper(II) complexes – Antibacterial activity. J. Therm. Anal. Calorim. 2008;92(1):245-251.
58. Dixit RB, Vanparia SF, Patel TS, Chandresh LJ, Doshi HV, Dixit BC. Synthesis and antimicrobial activities of sulfonohydrazide-substituted 8- hydroxyquinolin derivative and its oxinates. Appl. Organometal. Chem. 2010;24:408- 413.
59. Mastrolorenzo A, Scozzafava A, Supuran CT. Antifungal activity of silver andzinc complexes of sulfadrug derivatives incorporating arylsulfonylureido moieties. Eur. J Pharm. Sci. 2000;11:99–107.
60. Tavman A, Boz I, Birteksoz AS, Cinarli A. Spectral characterization an antimicrobial activity of Cu(II) and Fe(III) complexes of 2-(5-Cl/NO2-1H-benzimidazol-2- yl)-4- Br/NO2-phenols. J.Coord. Chem. 2010;63:1398-1410.
61. Arjmand F, Mohani B, Ahmad S. Synthesis, antibacterial, antifungal activity and interaction of CT-DNA with a new benzimidazole derived Cu(II) complex. Eur. J. Med. Chem. 2005;40:1103-1110.
62. Aghatabay NM, Neshat A, Karabiyik T, Somer M, Haciu M, Dulger DB, Synthesis, characterization and antimicrobial activity of Fe(II), Zn(II), Cd(II) and Hg(II) complexes with 2,6-bis(benzimidazol-2-yl) pyridine ligand. Eur.J. Med.Chem. 2007;42:205-213.
1. Collman JP, Gagne RR, Reed CA, Halbert TR, Lang G, Robinson WT, et al. Picket fence porphyrins. Synthetic models for oxygen binding hemoproteins. J Am Chem Soc. 1975;97:1427–39.
2. Schroeder HA, Balassa JJ, Tipton IH. Abnormal trace metals in man — nickel. J Chronic Dis. 1962;15(1):51–65.
3. Robert A, Eberle D, Kaplocoitz N. Role of glutathione in gastric mucosal cytoprotection. Am JPhysiol. 1984;247(3 Pt 1):296–304.
4. Hay RW. Inorganica Chimica Acta. Inorg Chim Acta. 1980;45:L83–4.5. Spike CG, Parry RW. Thermodynamics of Chelation. II. Bond Energy Effects in Chelate Ring Formation. J Am Chem Soc. 1953;75(15):3770–2.
6. Kasim ANM, Prabhu GV. Synthesis, Characterisation and Antimicrobial Screening of Aminobenzylated Mannich Bases of Acetamide and Urea. Asian J Chem. 2000;12(2):385–8.
7. Seigel H, Martin RB. Coordinating properties of the amide bond. Stability and structure of metal ion complexes of peptides and related ligands. Chem Rev. 1982;82(4):385–426.
8. Dyakar G, Lingaiah P. Synthesis and spectral studies of Ruthenium (III) complexes with amide group ligands. Asian J Chem. 1997;9(2):179–82.
9. Dyakar G, Lingaiah P. Studies on rhodium (III) complexes with ligands containing amide group. Indian J Chem. 1996;35A:614–6.
10. Senti F, Harker D. The Crystal Structure of Rhombohedral Acetamide. J Am Chem Soc. 1940;62(8):2008–2019.
11. Gerrard W, Lappert MF, Pyszora H, Wallis JW. Spectra and structure of amide complexes. J Chem Soc. 1960;p. 2144–51.
12. Kutzclnigg W, Mecke R. Spektroskopische Untersuchungen an organischen Ionen—V Die Struktur der Salze des Acetamids. Spectrochim Acta. 1962;18(4):549–60.
13. Penland RB, Mizushima S, Curran C, Quagliano JV. Infrared Absorption Spectra of Inorganic Coördination Complexes. X. Studies of Some Metal-Urea Complexes. J Am Chem Soc. 1957;79(7):1575–8.
14. Lippard SJ, Berg JM. Principles of Bioinorganic Chemistry. Mill Valley, CA: University Science Books; 1994.
15. Cowan JA. Inorganic Biochemistry/An introduction. Hoboken, NJ: Wiley-VCH Inc; 1997.
16. Wilkinson G, Gillard RD, Mc Cleverty JD. (Ed.) “Comprehensive coordination chemistry”, Pergamon, Oxford; 1987.
17. Mc Cleverty JA, Meyer TJ, “Comprehensive coordination chemistry II, from biology to nanotechnology” Elsevier, Amsterdam; 2003.
18. Cotton FA, Wilkinson G, Murillo CA, Bochmann M, “Advanced inorganic chemistry” 6th Edn., John Wiley and Sons, Inc; 1999.
19. Samy CR, Radhey S. Indian J. Chem. 1996;35A:1.
20. Shen X, Yang QLC, Xie Synth. React. Inorg. Met. Org. Chem. 1996;26:1135.
21. Kostova I. Recent patents on anti-cancer drug discovery. 2006;1:1.
22. Kostova I. Recent patents on anti-cancer drug discovery. 2006;1:1.
23. Allardyce CS, Dyson PJ. Platinum Metals Rev. 2001;45:62.
24. Abu-Surrah AS, Kettunen M. curr. Med. Chem. 2006;13:1337. Khan et al.; IRJPAC, 22(5): 12-23, 2021.
25. Clarke MJ. Coord. Chem Rev. 2003;23:209.
26. Zhang CX, Lippard SJ. Current Opinion in Chem. Bio. 2003;7:481.
27. Lions F, Martin FKV. J. Am. Chem. Soc. 1960;82:2733.
28. Greenwood NN, Earnshaw A, “Chemistry of Elements”, Pergamon; 1985.
29. Gerloch M, EC. Constable, “Transition metal chemistry”, VCH, Weinheim; 1994.
30. Clark MJ, Zhu F, Frasca DR. Chem. Rev. 1999;9:2511.
31. Guo Z, Sadler PJ. Angew Chem., Int Ed. 1999;11:1512.
32. Allardyce CS, Dorcier A, Scolaro C, Dyson PJ. Appl. Organomet. Chem. 2005;19:1.
33. Guo Z, Sadler PJ, Sykes AG. Adv. Inorg. Chem. 1999;49:183.
34. Di C, Milacic V, Frezza M, Ping Dou Q. Curr. Pharm. Des. 2009;7:777.
35. Bruijnincx PCA, Sadler PJ. Curr. Opin. Chem. Biol. 2008;2:197.
36. Dyson PJ, Sava G. Dalton Trans. 2006;16:1929.
37. Keppler BK, Berger MR, Klenner T, Heim ME. Adv. Drug. Res. 1990;19:243
38. Chohan ZH, Munawar A, Supuran CT. Metal Based Drugs. 2001;8:137.
39. Srivastava A, Singh NK, Singh SM. Biometals. 2003;16:311.
40. Hambley TW. Developing new metalbased therapeutics: Challenges and opportunities. Dalton Trans. 2007;43:4929- 4937.
41. Keppler BK, Berger MR, Klenner Th, Heim ME. Metal complexes as antitumor agents. Adv. Drug. Res. 1990;19:243.
42. Bregman H, Carroll PJ, Meggers E. Rapid access to unexplored chemical space by ligand scanning around a ruthenium center: Discovery of potent and selective protein kinase inhibitors. J. Am. Chem. Soc. 2006;128:877-884.
43. Riley DP, Lennon PJ, Neumann WL, Weiss RH. Toward the rational design of superoxide dismutase mimics: Mechanistic studies for the elucidation of substituent effects on the catalytic activity of macrocyclic manganese (II) complexes. J. Am. Chem. Soc. 1997;119:6522-5528.
44. Pacher P, Liaudet L, Bai PP, Mabley JG, Kaminski PM, Virag A, Deb L, Szabo E, Ungvari Wolin MS, Groves JT, Szabo C. Potent metalloporphyri peroxynitrite decomposition catalyst protects against the development of doxorubicininduced cardiac dysfunction. Circulation. 2003;107:896-904.
45. Hall MD, Dolman RC, Hambley TW. In metal complexes in tumor diagnosis and as anticancer agents, metal ions in biological systems, ed. A. Sigel and H. Sigel, Marcel Dekker, Inc., New York and Basel. 2004;24:297-322.
46. Bednarski PJ, Grunert R, Zielzki M, Wellner A, Mackay FS, Sadler PJ. Light activated destruction of cancer cell nuclei by platinum diazide complexes. Chem. Biol. 2006;13:61-67.
47. Fantus IG, Deragon G, Lai R, Tang S. Modulation of insulin action by vanadate: Evidence of a role for phosphotyrosine phosphatase activity to alter cellular signaling. Mol.Cell. Biochem. 1995;153:103-112.
48. Thompson TH, Orvig C. Vanadium in diabetes: 100 years from Phase 0 to Phase I. J. Inorg. Biochem. 2006;100:1925-1935.
49. Sessler JL, Seidel D. Synthetic expanded porphyrin chemistry. Angew. Chem. Int. Edit. 2003;42:5134-5175.
50. Hutchings SR , Song DZ, Fricker SP, Pang CCY. The ruthenium-based nitric oxide scavenger, AMD6221, augments cardiovascular responsiveness to noradrenaline in rats with streptozotocininduced diabetes. Eur. J. Pharmacol. 2005;528:132-136.
51. Musameh MD, Fuller BJ, Mann BE, Green CJ, Motterlini R. Positive inotropic effects of carbon monoxide-releasing molecules (CO-RMs) in the isolated perfused rat heart. Br. J.Pharmacol. 2006;149:1104- 1112.
52. Failes TW, Cullinane C, Diakos CI, Yamamoto N, Lyons JG, Hambley TW. Studies of a cobalt(III) complex of the MMP inhibitor marimastat: A potential hypoxiaactivated prodrug.Chem. Eur. J. 2007;13:2974-2982.
53. Weder JE, Dillon CT, Hambley TW, Kennedy BJ, Lay PA, Biffin JR, Regtop HL, Davies NM. Copper complexes of nonsteroidal anti-inflammatory drugs: an opportunity yet to be realized. Coord. Chem. Rev. 2002;232:95-126.
54. Kostowski W, Herman ZS. Farmakologia, Podstawyfarma, koterapiiWydawnic, lekarskie PZWL, Warszawa, Poland. 2003;62:270-272.
55. Blasco F, Perello L, Latorre J, Borras J, Garcia-GrandaCobalt S (II), Nikiel(II), Copper (II) complexes of sulfanilamide derivatives: Synthesis, spectroscopic studies, and antibacterial activity. Crystal structure of [Co(sulfacetamide) 2 (NCS)2]. J. Inorg. Biochem. 1996;61:143- 154.
56. Kremer E, Facchin G, Estevez E, Albores P, Baran EJ, Ellena J, Torre MH. Copper complexes with heterocyclic sulfonamides: Synthesis, spectroscopic characterization, microbiological and SOD-like activities: Crystal structure of [Cu(sulfisoxazole)2(H2O)4]*2H2O. J. Inorg. Biochem. 2006;100:1167-1175.
57. Olar R, Badea M, Carp O, Marinescu D, Lazar V, Balotescu C, Dumbrava A. Synthesis, characterisation and thermal behaviour of some thiosulfato- and sulfato copper(II) complexes – Antibacterial activity. J. Therm. Anal. Calorim. 2008;92(1):245-251.
58. Dixit RB, Vanparia SF, Patel TS, Chandresh LJ, Doshi HV, Dixit BC. Synthesis and antimicrobial activities of sulfonohydrazide-substituted 8- hydroxyquinolin derivative and its oxinates. Appl. Organometal. Chem. 2010;24:408- 413.
59. Mastrolorenzo A, Scozzafava A, Supuran CT. Antifungal activity of silver andzinc complexes of sulfadrug derivatives incorporating arylsulfonylureido moieties. Eur. J Pharm. Sci. 2000;11:99–107.
60. Tavman A, Boz I, Birteksoz AS, Cinarli A. Spectral characterization an antimicrobial activity of Cu(II) and Fe(III) complexes of 2-(5-Cl/NO2-1H-benzimidazol-2- yl)-4- Br/NO2-phenols. J.Coord. Chem. 2010;63:1398-1410.
61. Arjmand F, Mohani B, Ahmad S. Synthesis, antibacterial, antifungal activity and interaction of CT-DNA with a new benzimidazole derived Cu(II) complex. Eur. J. Med. Chem. 2005;40:1103-1110.
62. Aghatabay NM, Neshat A, Karabiyik T, Somer M, Haciu M, Dulger DB, Synthesis, characterization and antimicrobial activity of Fe(II), Zn(II), Cd(II) and Hg(II) complexes with 2,6-bis(benzimidazol-2-yl) pyridine ligand. Eur.J. Med.Chem. 2007;42:205-213.
63. Steinhilber D, Schubert-Zsilavecz MI, Roth HJ, Medizinische Chemie. Targets, Arzneistoffe, Chemische Biologie. 2 Auflage. Deutscher Apotheker Verlag, Stuttgart, Germany. 2010;13:593-596,
64. Willingham WM, Sorrenson JRJ. Physiologic role of copper complexes in antineoplasia. Trace Elem. Med. 1986;3:139-152.
65. Tapiero H, Townsend TM, Tew KD. Trace elements in human physiology and patology. Copper. Review. Biomed. Pharmacother. 2003;57:386-398.
66. Arnal N, Cristalli DO, de Alaniz MJT, Marra CA. Clinical utility of copper, ceruloplasmin and metallothionein plasma determinations in human neurodegenerative patients and their first-degree relatives. Brain Res. 2010;1319:118-130,
67. Molina JA, Jimenez-Jimenez FJ, Aguilar MV, Meseguer I, Mateos-Vega CJ, Gonzalez-Munoz MJ. Cerebrospinal fluid levels of transition metals in patients with Alzheimer’s disease, J. _eural Transm. 1998;105:479–488.
68. Magaki S, Raghavan R, Mueller C, Oberg KC, Vinters HV, Kirsch WM, Iron, copper and iron regulatory protein 2 in Alzheimer’s disease and related dementias.eurosci. Lett. 2007;418:72–76.
69. Ozcankaya R, Delibas N. Malondialdehyde, superoxide dismutase, melatonin, iron,copper, and zinc concentrations in patients with Alzheimer disease: cross-sectional study.Croat. Med. J. 2002;43:28–32.
70. Markesbery WR. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic.Biol. Med. 1997;23:134–147.
71. Forte G, Alimonti A, Violante N, Di Gregorio M, Senofonte O, Petrucci F, Sancesario GB. Bocca Calcium, copper, magnesium, silicon, and zinc content of hair in Parkinson’s disease, J.Trace Elem. Med. Biol. 2005;19:195–201.
72. Tuorkey MJF, Abdul-Aziz KK. A pioneer study on the anti-ulcer activities of copper nicotinate complex [CuCl(HNA)2] in experimental gastric ulcer induced by aspirinpyloris ligation model (Shay model). Biomed. & Pharmacother. 2009;63:194- 201.
73. Sharma S, Athar F, Maurya MR, Azam A. Copper(II) complexes with substituted thiosemicarbazones of thiophene-2- carboxaldehyde: Synthesis, characterization and antiamoebic activity against E. histolytica. Eur. J. Med. Chem. 2005;40:1414-1419.
74. Yasumatsu N, Yoshikawa Y, Adachi Y, Sakurai H. Antidiabetic copper(II)- picolinate: impact of the first transition metal in the metallopicolinate complexes. Bioorgan. Med. Chem. 2007;15:4917- 4922.
75. Veitia MS, Dumas F, Morgant G, Sorenson JR, Frapart Y, Tomas A. Synthesis, structural analysis and anticonvulsant activity of a ternary Cu(II) mononuclear complex containing 1,10-phenanthroline and the leading antiepileptic drug valproic acid. Biochimie. 2009;91:1286-1293.
76. Rainsford KD, Brune K, Whitehouse MW. Aspirin and related drugs: Their Actions and Uses. Birkhauser Verlag Basel und Stuttgart. 1977;109-117.
77. Pederson TC, Aust SD. The role of superoxide and singlet oxygen in lipid peroxidation promoted by xanthine oxidase. Biochem. Biophys. Res. Commun. 1973;52:1071- 1073.
78. Kovala-Demertzi D. Transition metal complexes of diclofenac with potentially interesting anti-inflammatory activity. J. Inorg. Biochem. 2000;79:153-157.
79. Suksrichavalit T, Prachayasittikul S, Nantasenamat Ch, Isarankura-NaAyudhya Ch, Prachayasittiku V. Copper complexes of pyridine derivatives with superoxide scavenging and antimicrobial activities. Eur. J. Chem. 2009;44:3259- 3265.
80. Rivero-Muller A, De Vizcaya-Ruiz A, Plant N, Ruiz L, Dobrota M. Mixed chelate copper complex, Casiopeina IIglyR, binds and degrades nucleic acids: Mechanism of cytotoxicity. Chem.- Biol. Interact. 2007;165:189-199.
81. Baquial JGL, Sorenson JRJ, Downregulation of NADPHdiaphorase (nitric oxide synthase) may account for the pharmacological activities of Cu(II)2(3,5- diisopropylasalicylate) 4. J Inorg. Biochem. 1995;60:133–148.
82. Sorenson JRJ. Copper complexes offer a physiological approach to treatment of chronic diseases. Prog. Med. Chem. 1989;26:437-568.
83. Sorenson JRJ, Soderberg LSF, Chang LW. Copper, iron, manganese and zink 3,5 diisopropylasalicylate complexes increase survival of gamma irradiated mice. Eur.J. Med. Chem. 1993;28:221– 229. 75. Greenaway FT, Hahn JJ, Xi N, Sorenson JRJ. Interaction of Cu(II) 3,5- diidopropylsalicylate with human serum albumin-an evaluation of spectroscopic data. Biometals. 1998;11:21–26.
84. Andreini C, Bertini I, Cavalarro G, Holliday GL, Thornton JM. Metal ions in biological catalysis: from enzyme databases to general principles. J. Biol. Inorg. Chem. 2008;13;1205-1218. 85. Hui Chao, Liang-Nian Ji,Co Cobalt Complexes as Potential Pharmaceutical Agents in: Gielen M.; Tiekink E.R.T. Metallotherapeutic drugs and metal-based diagnostic agents. The use of metals in medicine. John Wiley & Sons Ltd., Chichester, England.Chapter. 2005;11:201-218.
86. Ware DC, Palmer BD, Wilson WR, Denny WA. Hypoxia-selective antitumor agents. 7. Metal-complexes of aliphatic mustards as a new class of hypoxia- selective cytotoxins – Synthesis and evaluation of cobalt(III) complexes of bidentate mustards. J Med. Chem. 1993;36:1839- 1846.
87. Ott I, Kircher BR. Gust, Investigations on the effects of cobalt-alkyne complexes on leukemia and lymphoma cells: cytotoxicity and cellular uptake. J. lnorg. Biochem. 2004;98;485-489.
88. Lopez-Sandoval H, Londono-Lemos ME, Garza-Velasco R, Poblano-Melendez I, Granada-Macias P, Gracia-Mora I, Barba-Behrens N. Synthesis, structure and biological activities of cobalt(II) and zinc(II) coordination compounds with 2- benzimidazole derivatives. J. Inorg.Biochem. 2008;102:1267-1276.
89. Tuberculosis -TB Guidelines – Treatment”. cdc. gov. Centers for Disease Control; 2016.
90. Ogunniran KO, Ajanaku KO, James OO, Ajani OO, Adekoya JA, Nwinyi OC. “Synthesis, characterization, antimicrobial activity and toxicology study of some metal complexes of mixed antibiotics”. Afri., J. Pure and Appli.,Chem. 2008;2(7):69- 74.
91. Heater SJ, Carrano MW, Rains D, Walter RB, Ji D, Yan Carrano Q, CJ. Interaction of oxo-bridged vanadium (III) phenanthroline and bipyridine dimers with DNA. Inorganic chemistry. 2000;39(17):3881-3889.
92. Srivastava RS. Synthesis, characterization and fungitoxicity of bidentate high- spin six coordinate 3d metal complexes with N-(5- phenyl-1, 3, 4- thiadiazol-2-yl) aceta/benzamidines. Inorganica Chimica Acta, 55, L71-L74; 1981.
93. Zommer S, Lipiec T. determination of isonicotinic acid hydrazide in various substances and tablets with cu2+ ions in the presence of acetone. Acta poloniae pharmaceutica. 1963;20:229-232.
94. Munson JW, Connors KA. Spectrophotometric determination of acid hydrazidesvianickel (II)‐catalyzed hydroxamic acid formation. Journal of Pharmaceutical Sciences. 1972;61(2):211- 213.
95. Debbie C. Crans, Kateryna Kostenkova. Open questions on the biological roles of first-row transition metals, Communications Chemistry. 2020;3: 104.
96. Albert A. Mode of action of isoniazid. Nature, Lond. 1956;525-526.
97. Victor W, Rodwell W, David Bender A, Kathleen Botham M. J. Peter Kennelly, P. Anthony Weil, The Biochemical Roles of Transition Metals; 2020
64. Willingham WM, Sorrenson JRJ. Physiologic role of copper complexes in antineoplasia. Trace Elem. Med. 1986;3:139-152.
65. Tapiero H, Townsend TM, Tew KD. Trace elements in human physiology and patology. Copper. Review. Biomed. Pharmacother. 2003;57:386-398.
66. Arnal N, Cristalli DO, de Alaniz MJT, Marra CA. Clinical utility of copper, ceruloplasmin and metallothionein plasma determinations in human neurodegenerative patients and their first-degree relatives. Brain Res. 2010;1319:118-130,
67. Molina JA, Jimenez-Jimenez FJ, Aguilar MV, Meseguer I, Mateos-Vega CJ, Gonzalez-Munoz MJ. Cerebrospinal fluid levels of transition metals in patients with Alzheimer’s disease, J. _eural Transm. 1998;105:479–488.
68. Magaki S, Raghavan R, Mueller C, Oberg KC, Vinters HV, Kirsch WM, Iron, copper and iron regulatory protein 2 in Alzheimer’s disease and related dementias.eurosci. Lett. 2007;418:72–76.
69. Ozcankaya R, Delibas N. Malondialdehyde, superoxide dismutase, melatonin, iron,copper, and zinc concentrations in patients with Alzheimer disease: cross-sectional study.Croat. Med. J. 2002;43:28–32.
70. Markesbery WR. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic.Biol. Med. 1997;23:134–147.
71. Forte G, Alimonti A, Violante N, Di Gregorio M, Senofonte O, Petrucci F, Sancesario GB. Bocca Calcium, copper, magnesium, silicon, and zinc content of hair in Parkinson’s disease, J.Trace Elem. Med. Biol. 2005;19:195–201.
72. Tuorkey MJF, Abdul-Aziz KK. A pioneer study on the anti-ulcer activities of copper nicotinate complex [CuCl(HNA)2] in experimental gastric ulcer induced by aspirinpyloris ligation model (Shay model). Biomed. & Pharmacother. 2009;63:194- 201.
73. Sharma S, Athar F, Maurya MR, Azam A. Copper(II) complexes with substituted thiosemicarbazones of thiophene-2- carboxaldehyde: Synthesis, characterization and antiamoebic activity against E. histolytica. Eur. J. Med. Chem. 2005;40:1414-1419.
74. Yasumatsu N, Yoshikawa Y, Adachi Y, Sakurai H. Antidiabetic copper(II)- picolinate: impact of the first transition metal in the metallopicolinate complexes. Bioorgan. Med. Chem. 2007;15:4917- 4922.
75. Veitia MS, Dumas F, Morgant G, Sorenson JR, Frapart Y, Tomas A. Synthesis, structural analysis and anticonvulsant activity of a ternary Cu(II) mononuclear complex containing 1,10-phenanthroline and the leading antiepileptic drug valproic acid. Biochimie. 2009;91:1286-1293.
76. Rainsford KD, Brune K, Whitehouse MW. Aspirin and related drugs: Their Actions and Uses. Birkhauser Verlag Basel und Stuttgart. 1977;109-117.
77. Pederson TC, Aust SD. The role of superoxide and singlet oxygen in lipid peroxidation promoted by xanthine oxidase. Biochem. Biophys. Res. Commun. 1973;52:1071- 1073.
78. Kovala-Demertzi D. Transition metal complexes of diclofenac with potentially interesting anti-inflammatory activity. J. Inorg. Biochem. 2000;79:153-157.
79. Suksrichavalit T, Prachayasittikul S, Nantasenamat Ch, Isarankura-NaAyudhya Ch, Prachayasittiku V. Copper complexes of pyridine derivatives with superoxide scavenging and antimicrobial activities. Eur. J. Chem. 2009;44:3259- 3265.
80. Rivero-Muller A, De Vizcaya-Ruiz A, Plant N, Ruiz L, Dobrota M. Mixed chelate copper complex, Casiopeina IIglyR, binds and degrades nucleic acids: Mechanism of cytotoxicity. Chem.- Biol. Interact. 2007;165:189-199.
81. Baquial JGL, Sorenson JRJ, Downregulation of NADPHdiaphorase (nitric oxide synthase) may account for the pharmacological activities of Cu(II)2(3,5- diisopropylasalicylate) 4. J Inorg. Biochem. 1995;60:133–148.
82. Sorenson JRJ. Copper complexes offer a physiological approach to treatment of chronic diseases. Prog. Med. Chem. 1989;26:437-568.
83. Sorenson JRJ, Soderberg LSF, Chang LW. Copper, iron, manganese and zink 3,5 diisopropylasalicylate complexes increase survival of gamma irradiated mice. Eur.J. Med. Chem. 1993;28:221– 229. 75. Greenaway FT, Hahn JJ, Xi N, Sorenson JRJ. Interaction of Cu(II) 3,5- diidopropylsalicylate with human serum albumin-an evaluation of spectroscopic data. Biometals. 1998;11:21–26.
84. Andreini C, Bertini I, Cavalarro G, Holliday GL, Thornton JM. Metal ions in biological catalysis: from enzyme databases to general principles. J. Biol. Inorg. Chem. 2008;13;1205-1218. 85. Hui Chao, Liang-Nian Ji,Co Cobalt Complexes as Potential Pharmaceutical Agents in: Gielen M.; Tiekink E.R.T. Metallotherapeutic drugs and metal-based diagnostic agents. The use of metals in medicine. John Wiley & Sons Ltd., Chichester, England.Chapter. 2005;11:201-218.
86. Ware DC, Palmer BD, Wilson WR, Denny WA. Hypoxia-selective antitumor agents. 7. Metal-complexes of aliphatic mustards as a new class of hypoxia- selective cytotoxins – Synthesis and evaluation of cobalt(III) complexes of bidentate mustards. J Med. Chem. 1993;36:1839- 1846.
87. Ott I, Kircher BR. Gust, Investigations on the effects of cobalt-alkyne complexes on leukemia and lymphoma cells: cytotoxicity and cellular uptake. J. lnorg. Biochem. 2004;98;485-489.
88. Lopez-Sandoval H, Londono-Lemos ME, Garza-Velasco R, Poblano-Melendez I, Granada-Macias P, Gracia-Mora I, Barba-Behrens N. Synthesis, structure and biological activities of cobalt(II) and zinc(II) coordination compounds with 2- benzimidazole derivatives. J. Inorg.Biochem. 2008;102:1267-1276.
89. Tuberculosis -TB Guidelines – Treatment”. cdc. gov. Centers for Disease Control; 2016.
90. Ogunniran KO, Ajanaku KO, James OO, Ajani OO, Adekoya JA, Nwinyi OC. “Synthesis, characterization, antimicrobial activity and toxicology study of some metal complexes of mixed antibiotics”. Afri., J. Pure and Appli.,Chem. 2008;2(7):69- 74.
91. Heater SJ, Carrano MW, Rains D, Walter RB, Ji D, Yan Carrano Q, CJ. Interaction of oxo-bridged vanadium (III) phenanthroline and bipyridine dimers with DNA. Inorganic chemistry. 2000;39(17):3881-3889.
92. Srivastava RS. Synthesis, characterization and fungitoxicity of bidentate high- spin six coordinate 3d metal complexes with N-(5- phenyl-1, 3, 4- thiadiazol-2-yl) aceta/benzamidines. Inorganica Chimica Acta, 55, L71-L74; 1981.
93. Zommer S, Lipiec T. determination of isonicotinic acid hydrazide in various substances and tablets with cu2+ ions in the presence of acetone. Acta poloniae pharmaceutica. 1963;20:229-232.
94. Munson JW, Connors KA. Spectrophotometric determination of acid hydrazidesvianickel (II)‐catalyzed hydroxamic acid formation. Journal of Pharmaceutical Sciences. 1972;61(2):211- 213.
95. Debbie C. Crans, Kateryna Kostenkova. Open questions on the biological roles of first-row transition metals, Communications Chemistry. 2020;3: 104.
96. Albert A. Mode of action of isoniazid. Nature, Lond. 1956;525-526.
97. Victor W, Rodwell W, David Bender A, Kathleen Botham M. J. Peter Kennelly, P. Anthony Weil, The Biochemical Roles of Transition Metals; 2020


